In the realm of molecular biology and biochemistry, streptavidin stands out as a crucial player, providing researchers with a powerful tool for various applications. This small yet mighty protein has become an indispensable asset in laboratories worldwide, owing to its unique properties and versatile uses.
Understanding Streptavidin:
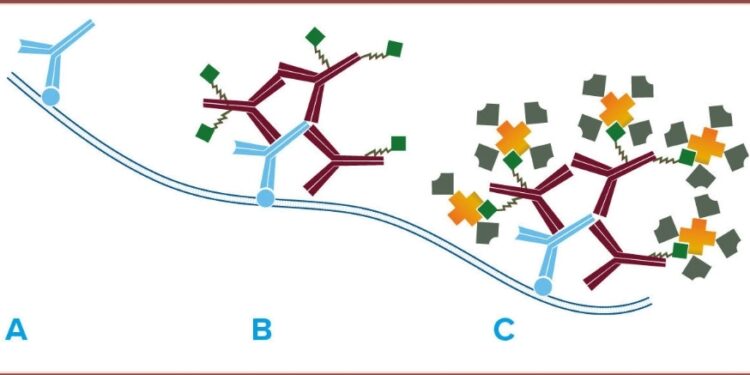
Streptavidin is a tetrameric protein, meaning it is composed of four subunits. It was first isolated from the bacterium Streptomyces avidinii, which naturally produces avidin, a protein closely related to streptavidin. What makes streptavidin particularly noteworthy is its exceptionally strong binding affinity for biotin, a water-soluble B-vitamin.
The Biotin-Binding Affinity:
Streptavidin’s interaction with biotin is one of the tightest non-covalent binding interactions known in nature. The dissociation constant (Kd) for streptavidin-biotin binding is in the order of 10^-14 M, making it an incredibly stable and specific interaction. This high affinity has paved the way for the development of numerous applications across various scientific disciplines.
Applications in Molecular Biology:
- Affinity Chromatography: Streptavidin is commonly immobilized on solid supports, such as agarose or magnetic beads. Biotinylated molecules, such as proteins or nucleic acids, can then be selectively captured and separated from complex mixtures using affinity chromatography. This technique is widely used for purifying and isolating specific biomolecules with high purity.
- Biotin-Streptavidin Detection Systems: Streptavidin plays a pivotal role in numerous detection methodologies, such as Western blotting and enzyme-linked immunosorbent assays (ELISA). In these scenarios, streptavidin forms conjugates with enzymes or fluorescent dyes, facilitating the observation and measurement of target molecules specifically marked with biotin.
Applications in Cell Biology:
- Fluorescent Imaging: Streptavidin-fluorophore conjugates are extensively employed in fluorescence microscopy to visualize cellular structures and specific biomolecules. By using biotinylated probes, researchers can precisely label and track cellular components with high specificity.
- Cell Surface Labeling: Biotin-streptavidin interactions are exploited to label cell surfaces for various purposes, including flow cytometry and cell sorting. This method enables the identification and isolation of specific cell populations based on surface markers.
Applications in Medical Diagnostics:
- Diagnostic Assays: Streptavidin is a crucial component in various diagnostic assays, contributing to the specificity and sensitivity of tests. Biotinylated probes or antigens can be immobilized on surfaces, and the binding of streptavidin-conjugated enzymes or markers enhances the detection of target molecules.
- In Vivo Imaging: Streptavidin-based imaging agents are employed in in vivo imaging studies. By conjugating streptavidin to imaging probes, researchers can target and visualize specific tissues or cells in living organisms, facilitating non-invasive imaging techniques.
- such as Western blotting and enzyme-linked immunosorbent assays (ELISA). In these scenarios, streptavidin forms conjugates with enzymes or fluorescent dyes, facilitating the observation and measurement of target molecules specifically marked with biotin.
Conclusion:
Streptavidin’s exceptional biotin-binding affinity has propelled it to the forefront of molecular biology and biotechnology. The adaptability, robustness, and precision inherent in streptavidin render it an indispensable asset across a diverse spectrum of uses, spanning from foundational scientific inquiries to diagnostic applications in the medical field. As advancements in technology persist, the potential for streptavidin to play an increasingly expansive role grows, fostering breakthroughs in diverse scientific realms and enhancing our comprehension of the intricate landscape of biomolecular interactions.


 Home
Home