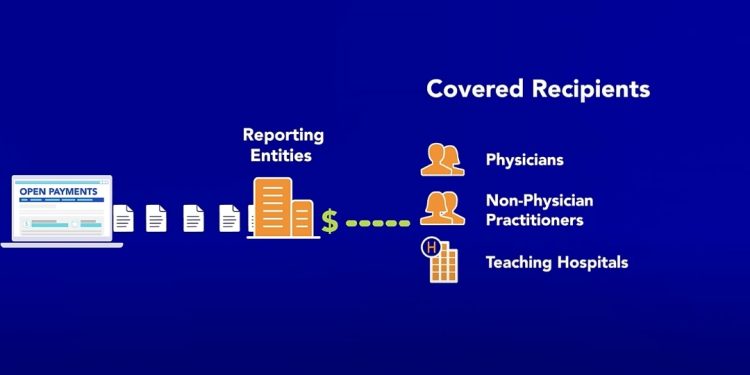
To increase transparency and accountability, the Centers for Medicare & Medicaid Services (CMS) established the U.S Sunshine Act, followed by the Open Payments program.
The law and the CMS mandate that certain entities, like drug and medical device manufacturers or life sciences companies, disclose payments and transfers of value (TOVs) made to covered recipients, primarily physicians and teaching hospitals.
All Open Payments reports are to be submitted by 31st March, which is also the submission deadline. This entire process of collecting, analyzing, and validating open payment reports can be extremely challenging.
Organizations generate massive amounts of data throughout the year, and then reporting all this transactional data to the CMS in a specified format, ensuring accuracy and timeliness, requires extensive efforts, resources, and more.
Still, by leveraging a data-driven compliance platform and ensuring proactiveness, life sciences companies prepare transparency reports before the deadline. This blog gives away the seven critical checks to perform before CMS submission and more.
It focuses on establishing the importance of ensuring compliance with CMS reporting obligations, discusses the seven checks that need to be performed, and provides readers with actionable insights on how they can enhance the effectiveness and efficiency of their transparency reporting program.
Why is Adherence to the U.S. Sunshine Act an Imperative for Life Sciences Companies?
Open Payments empowers patients by granting them access to valuable information about potential financial relationships between their healthcare providers and industry players. This transparency fosters trust and helps patients make informed decisions about their care.
While Open Payments empowers patients through transparency, its core purpose lies in its alignment with the broader goals of the Centers for Medicare & Medicaid Services (CMS) and the Department of Health and Human Services (HHS).
These agencies consider Open Payments a crucial tool to combat potential conflicts of interest, foster ethical interactions, and prevent fraud and abuse within the life sciences and healthcare industry.
Here are a few ways through which Open Payments Reporting aligns with regulatory objectives:
- Reducing Healthcare Costs
- Identifying inappropriate prescribing: By transparentizing financial relationships between physicians and life sciences companies, Open Payments helps identify potential patterns of overprescribing or prescribing based on financial incentives rather than patient needs. This lowers the overall healthcare cost.
- Combating fraud and abuse: Transparency discourages illegal kickback schemes and other fraudulent practices that drive up healthcare costs. Open Payments data can be used to detect and investigate such activities.
- Protecting Patient Safety
- Promoting informed decision-making: Patients with knowledge of potential financial ties can make more informed choices about their care.
- Identifying conflicts of interest: Open Payments data allows healthcare providers to be aware of potential conflicts of interest and make informed decisions prioritizing patient well-being over financial incentives.
- Ensuring Fair Market Value:
- Preventing price gouging: Transparency on physician payments helps ensure that drug and device prices are based on fair market value rather than inflated prices to compensate for financial incentives.
- Leveling the playing field: Open Payments encourage fair competition within the life sciences industry by reducing the influence of financial relationships on prescribing decisions.
Consequences of Non-Compliance
Regulators actively monitor compliance with Open Payments. Failing to accurately and timely report data can result in:
- Financial penalties: Up to $1,150,000 per violation.
- Reputational damage: Public disclosure of non-compliance can erode trust with patients, healthcare providers, and the public.
- Exclusion from government healthcare programs: In extreme cases, non-compliant companies may face exclusion from participating in Medicare and Medicaid programs.
Therefore, adhering to Open Payments is a legal requirement and a strategic imperative for life sciences companies.
By embracing transparency and ethical practices, companies can foster trust, mitigate regulatory risks, and ultimately contribute to a more efficient, patient-centered healthcare system.
Other reasons why adhering to CMS reporting obligations is essential:
- It fosters trust and ethical practices: Open Payments promotes integrity and ethical interactions within the healthcare system.
- It protects patients: Transparency empowers patients to make informed decisions about their care based on potential conflicts of interest.
- It avoids hefty penalties: Failing to accurately and timely report CMS data can result in fines of up to $1,150,000 per violation.
So, how do you avoid such pitfalls?
Before pressing the submit button, take a deep breath and run through these seven last checks:
The 7 Last Checks to Perform Before CMS Submission
- Mid-Level Practitioners: Include payment data to physician assistants, nurse practitioners, and other covered non-physician practitioners. CMS increased the list of covered recipients; therefore, staying up to date with it is crucial for compliant reporting.
- Checks for Duplication: Double-check for and eliminate duplicate entries to ensure accurate reporting.
- Data Inclusion Timeline: Only include data from 1st January to 31st December of the reporting year. Avoid including additional or missing information.
- Including The Late Entries: Gather data for confirmed December 2023 transactions even if reported in January 2024.
- Global Currency Conversion: Think globally, act locally! Convert all international transactions to USD using the appropriate exchange rate.
- Zero Dollar Transactions: Investigate anomalies. Analyze and address negative or $0 transactions to ensure data accuracy.
- Mass Scale Transactions: Be meticulous! Carefully categorize and report large-scale transactions to avoid misinterpretations.
Compliance promotes ethical healthcare practices and demonstrates an organization’s adherence to applicable regulatory compliance requirements, enabling life sciences companies to avoid regulatory and board scrutiny.
By implementing these last checks, you can confidently submit your Open Payments reports, knowing you’ve played your part in fostering a transparent and accountable healthcare system.
Additionally, utilizing the valuable resources on the CMS Open Payments website, including FAQs, webinars, and data dictionary guides, will equip you with the knowledge and tools to navigate the reporting process seamlessly.
You can also leverage a data-driven CMS Open Payments Reporting solution to increase the effectiveness of your transparency reporting program and efficiently submit spend reports to the CMS.
Leveraging technology such as artificial intelligence and automation has proven to be one of the best ways to streamline open payments reporting and identify and remediate risks associated with CMS Open Payments Reports.


 Home
Home