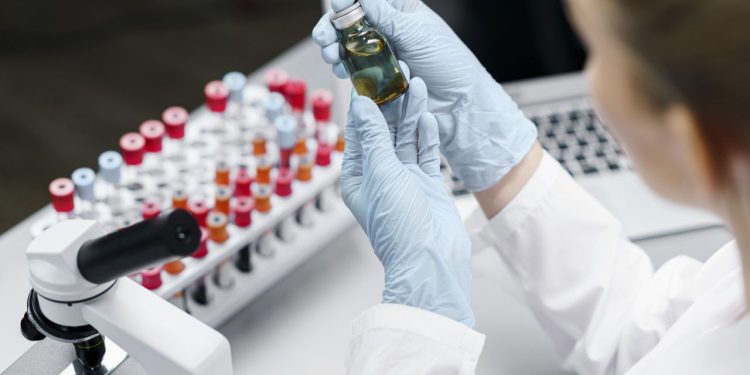
CQAs are defined as the physical, chemical, biological, or microbiological properties or characteristics that need to be within an appropriate limit, range, or distribution to ensure the desired product quality. Identifying CQAs early in the drug development process is crucial, as these attributes can significantly affect the final drug product’s safety and efficacy. The determination of CQAs is a dynamic process, evolving as product knowledge and understanding of the manufacturing process increase. It involves a thorough risk assessment where variables such as potency, purity, stability, and bioavailability are evaluated to discern their impact on the drug product.
The identification of CQAs is guided by a thorough understanding of the therapeutic context of the drug, its mechanism of action, and the inherent properties of the molecule itself. Techniques such as High-Performance Liquid Chromatography (HPLC), Mass Spectrometry (MS), and Nuclear Magnetic Resonance (NMR) spectroscopy are commonly employed in this phase to provide a detailed characterization of the molecule.
Read also: GMP biologics characterization & release testing
Analytical Method Development: The Pathway to Precision
Once CQAs have been determined, the focus shifts to the development of analytical methods tailored to these attributes. Analytical method development is a systematic process designed to ensure that an analytical method is suitable for its intended purpose. It encompasses the design, optimization, and validation of methods that can reliably quantify, identify, and assess the quality of the drug substance or product.
The development of robust analytical methods is critical for several reasons. First, it facilitates the precise quantification of CQAs, allowing for the accurate assessment of product quality. Secondly, it supports the establishment of specifications and process controls. Finally, it is essential for regulatory submissions, as regulatory agencies require comprehensive data demonstrating that the analytical methods used are suitable for their intended purpose.
Method development involves several stages, including method selection, method optimization, and method validation. During method selection, the most appropriate analytical technique is chosen based on the CQA to be analyzed. Method optimization then fine-tunes the selected method to achieve the best performance, adjusting parameters such as column type, mobile phase composition, and detector settings. Finally, method validation confirms that the method performs reliably under different conditions and is capable of producing consistent, reproducible results.


 Home
Home